Lupin Pharmaceuticals has issued a recall order for a lot of Mibelas 24 Fe birth control pills. The lot in question was the L600518, expiring in May 2018.
It appears that the pills were subjected to a packaging error, where the blister was rotated, and the orientation of the tablets was altered. The error would cause the first pills to not serve as a birth control method.

Additionally, the expiration date was no longer visible on the blister.
Recalling to avoid unintended pregnancies
Mibelas 24 Fe packets come with 28 tablets. 24 of them comprised of active ingredients and are labeled with “LU/N81,” the four others are comprised of inert ingredients and labeled “LU/M22.” It is available in all of the United States to wholesale pharmacies and clinics.
According to the Food and Drug Administration (FDA), the packaging error caused the pills to be put in the wrong order, increasing the risk of unintended pregnancy. Neither new or previous users may not become aware of the error, which could result in the pills being consumed in the wrong order.
Lupin Pharmaceuticals has warned its distributors and started a recall order on the affected products. The company can be reached at 1-800-399-2561 from 8 a.m. to 5 p.m.
Doctors usually prescribe Mibelas 24 Fe for the patient to take on the first day of their menstrual period. The pills should be taken daily, taking one white pill in 24-hour intervals.
Indications for Mibelas 24 Fe explain that the pills should be swallowed or chewed. The patient should also not skip taking a pill for over 24 hours.
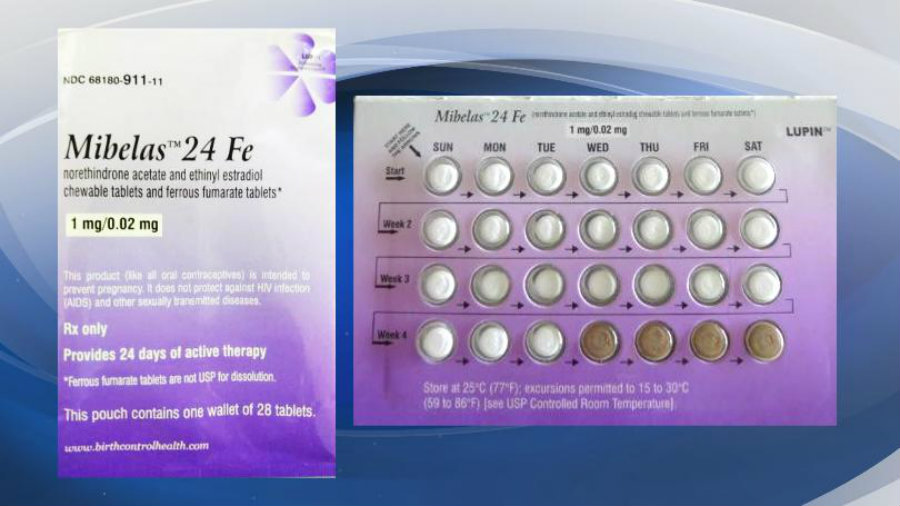
Despite the recall order, the packaging indicates that Mibelas 24 Fe should not be considered an “effective contraceptive” until after the first seven consecutive days of taking the pills.
When taken correctly, birth control pills are 99.9 percent efficient. One of the few drawbacks is that the pill does not protect the patient against sexually transmitted diseases.
Birth control pills work by supplying the body with hormones to inhibit its natural cycle of fertility, causing the woman to stop ovulating. Other contraceptives act on the cervical mucus to sabotage the sperm’s ability to find the egg.
Not all birth control pills require being taken daily. One example is Seasonale, which reduces the number of menstrual periods to four a year. The product achieves this by using two different types of hormones. It requires the patient to take the pill for 12 weeks and then go on for a week with inactive pills.
Most birth control pills come with something between 21 and 28 pills, and most of them provide the patient with placebo pills.
One should always consult a doctor regarding the use of birth control pills. Women older than 35 that smoke should not take birth control. They should also not have suffered from blood clots, serious heart or liver disease, breast or uterus cancer, or uncontrolled high blood pressure. Other drugs such as antibiotics may reduce the effects of the pill, which is why a specialist should always oversee their use.
Source: FDA
