The Food and Drug Administration warned this Wednesday that some blood tests may have significantly underestimated lead levels. The FDA along with the Centers for Disease Control and Prevention urged the population to retest children, pregnant and breastfeeding women who tested their blood with the faulty test.
The FDA and the CDC said the tests were made by Magellan Diagnostics, a testing company from Massachusetts. Magellan Diagnostics products are used in labs and doctors’ offices throughout the United States. The institutions noted that the problem could go as far back as 2014. The warning only applies to tests in which blood samples were taken from a vein, not for the more common tests in which fingers or heels are pricked for a blood sample.
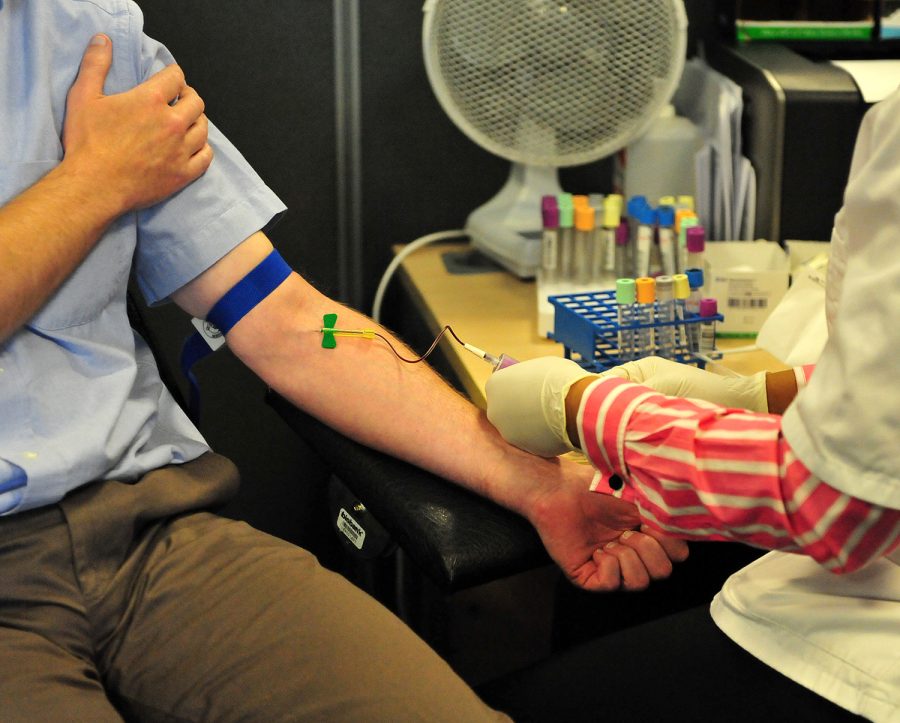
Mangella tests show underestimated lead levels
The FDA published a Safety Communication statement on its website, where they said that people should follow their recommendations instead of the ones issued by Magellan Diagnostics on April 28.
“Magellan Diagnostics’ LeadCare Testing Systems may underestimate BLLs and give inaccurate results when processing venous blood samples,” said the FDA in its statement. “Falsely lower test results may lead to improper patient management and treatment for lead exposure or poisoning.”
The FDA and the CDC recommended that children younger than six years old be retested if their Magellan venous tests showed lead levels at less than ten micrograms per deciliter. They added that women who are pregnant or breastfeeding should get retested too, while other adults concerned about lead exposure should talk to their doctors about a possible repeat test.
Over 400,000 to 600,000 venous blood tests are conducted every year using Magellan technology, compared to more than 4 million finger or heel stick tests, said John Kraeutler, chief executive officer of Meridian Bioscience, Inc., the company that owns Magellan. According to Kraeutler, Magellan is working closely with the FDA to solve the situation and is also working on moving customers to finger-stick tests from vein-based tests.
Mangellan contacted the FDA to assess the damage in faulty blood tests
According to the FDA’s statement, Magellan initially recognized the possible problem during the performance of their LeadCare Ultra –one of the trials- in August 2014 and they notified customers by letter on November 2014. The company instructed facilities to implement a 24-hour incubation step with the blood samples to mitigate and resolve what they noted was a low risk of underestimation of BLL (blood lead levels).
On November 4, 2016, Magellan notified its customers of a similar problem when processing venous blood samples taken with their LeadCare II testing systems, and by November 11 they notified customers by bulletin that the rubber caps of some of the blood collection tubes may introduce a substance into the blood sample when used with the LeadCare II systems. In both cases, they recommended implementing a minimum 4-hour incubation step with the samples.
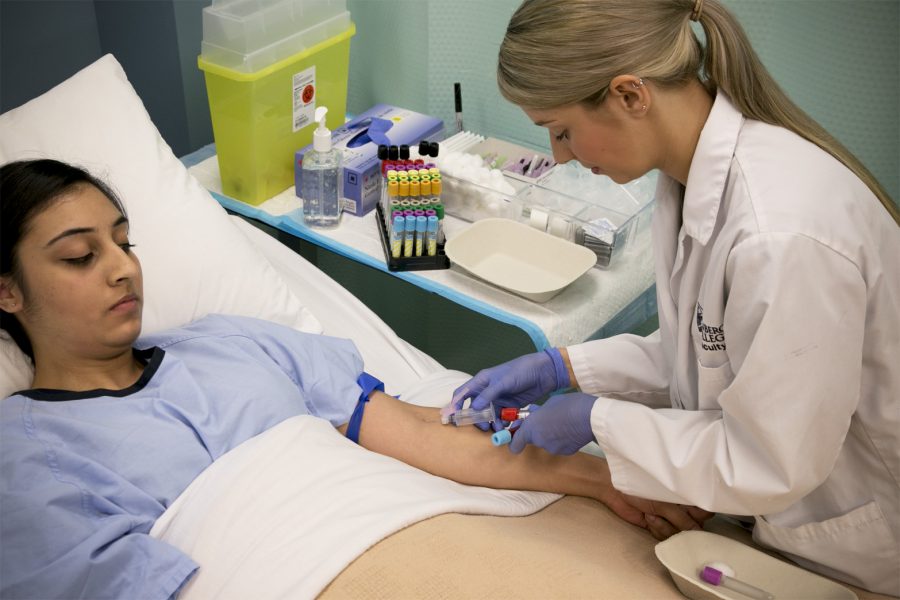
However, on April 28, Magellan notified customers they should no longer use the blood collection tubes, and that they should also discontinue the 24-hour incubation method. At that time, the FDA involved, and they began investigating the products to find the inaccuracy of the tests results.
Patrick Breysse, director of the CDC’s National Center for Environmental Health, said that the CDC is keeping a close eye on communities like Flint, Michigan, where lead has contaminated the water. Breysse estimated that “less than 1 percent” of children in Flint might be at risk for having lead levels that were reported using inaccurate tests.
Lead exposure in children can cause cognitive impairments
Tim Hill, an official at the Centers for Medicare and Medicaid Services, said that Medicaid would pay for the retesting of children on the program who were tested with Magellan systems, according to The Washington Post. He added that people covered by private insurance should ask their health plan about taking another lead test.
Lead testing in children is typically done by a finger or heel stick known as capillary test. If such results find elevated lead levels, the results are confirmed through a venous blood test. The FDA noted that it hadn’t found any evidence of problems with the capillary blood samples.
“There is no safe level of lead exposure for children, and the best ‘treatment’ for lead poisoning is to prevent lead exposure before it happens,” said Jennifer Lowry, chair of the American Academy of Pediatrics Council on Environmental Health, according to The Washington Post.
The CDC noted that 4 million households have children that are exposed to high levels of lead. Lead can impair cognitive abilities and cause other damage in children.
Source: FDA
