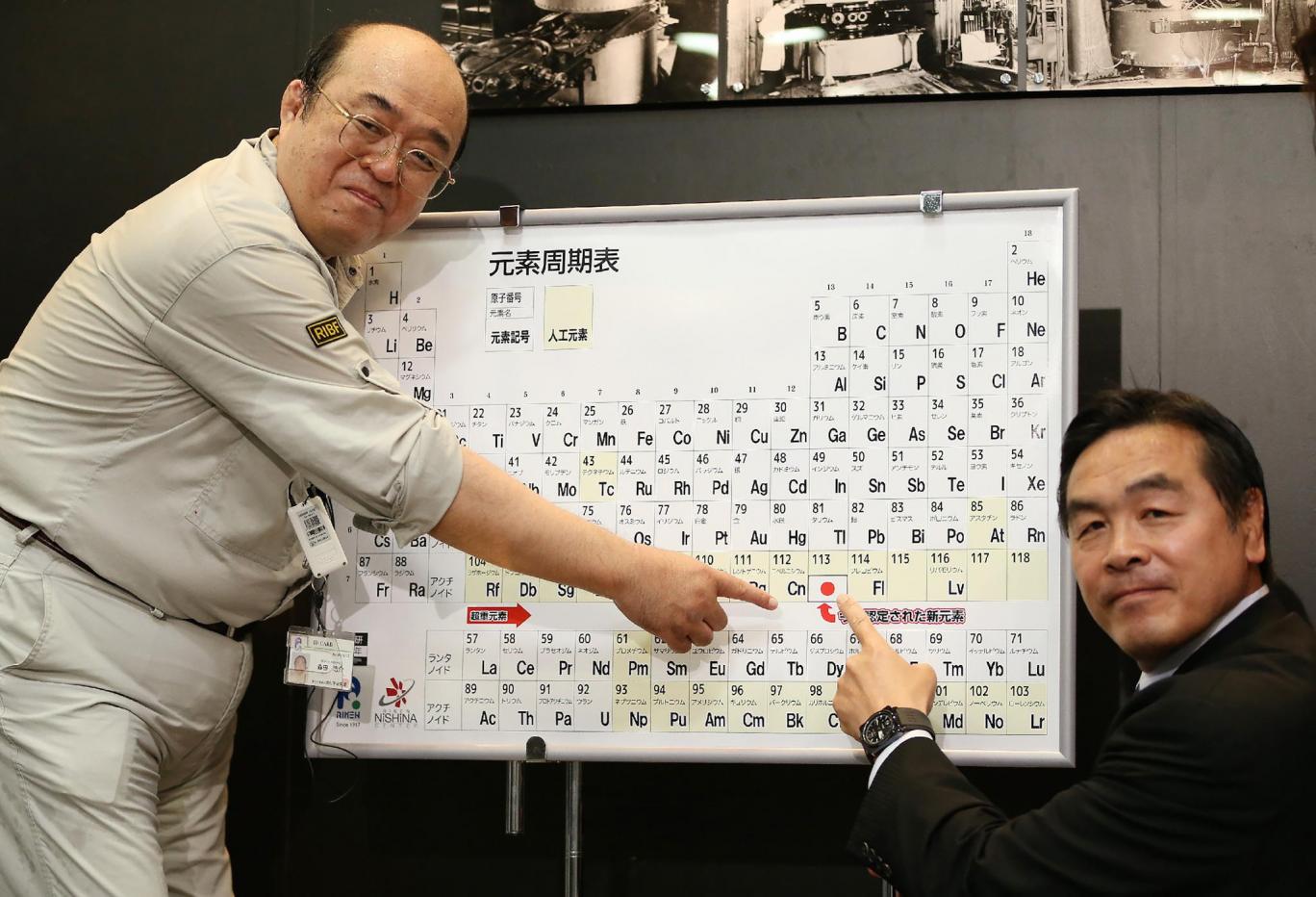
The periodic table has just been enlarged last Wednesday, when the International Union of Pure and Applied Chemistry approved the names and symbols of four new elements, after a five-month review.
The new elements are nihonium (Nh), moscovium (Mc), tennessine (Ts), and oganesson (Og). They were first verified last December. In June 2016, the IUPAC suggested names for the elements, which entered later on a review. All of the names make reference to a place, for example, “moscovium” to Moscow and “tennessine” to Tennessee.
“Overall, it was a real pleasure to realize that so many people are interested in the naming of the new elements, including high-school students, making essays about possible names and telling how proud they were to have been able to participate in the discussions,” said Jan Reedijk, the president of the Inorganic Chemistry Division at IUPAC.
What are these new elements?
Elements such as oxygen, hydrogen or carbon are easily found in nature. By contrast, nihonium (Nh), moscovium (Mc), tennessine (Ts), and oganesson (Og) are synthetic elements, which means that they are not found naturally in the atmosphere. Instead they are artificially created.
These chemical elements have a peculiar condition since they only exist for fractions of a second before they transform into other elements in the lab. They are some of the heaviest metals on the table. They were added to the seventh period or row on the table.
No elements had been added to the periodic table since 2011, when members of the heavy metal band livermorium (element 116) and flerovium (element 114) were added to it. Adding, discovering, and naming new elements is not something that scientists take slightly. It takes a lot of work and even cooperation to do so.
Nh, Mc, Ts, and Og were officially designated as elements 113, 115, 117, and 118, respectively.
“It is a long process from initial discovery to the final naming, and IUPAC is thankful for the cooperation of everyone involved,” said Jan Reedijk
How is the naming process for a chemical element?
Finding a new element is a hard work for a scientist. Therefore, when such an infrequent event occurs, it is perfectly understandable that chemists take their time choosing a name for it.
The naming process is not entirely open to the public, as the IUPAC explains. Certainly, a lot of institutions and groups can make petitions, suggestions or comments about the name of the element. However, only the discoverers are allowed to propose names and symbols for the element.
Regarding these four new elements, IUPAC asked the discoverers to name them only after a place, scientist, property, mineral, or mythological concept. For example, Oganesson honors Armenian nuclear physicist and element hunter Yuri Oganessian, known as the “grandfather of superheavy elements.”
Nihonium or element 113 is a highly radioactive element with an extremely short half-life. It is also the first element to be ever discovered by Japanese scientists. That is why, its name comes from Japan’s name in Japanese “‘nihon,” which means literally “the land of the rising sun”.
Source: The Christian Science Monitor
