The U.S. Food and Drug Administration approved Radicava (edaravone) to treat patients with amyotrophic lateral sclerosis (ALS) on Friday. The disease, also known as Lou Gehrig’s disease, affects and kills the nerve cells that control voluntary muscles.
The National Institute of Neurological Disorders and Stroke (NINDS) says that voluntary functions affected by ALS include chewing, walking, breathing and talking. ALS is a progressive disease, which means that symptoms get worse over time. There is no cure for ALS and no treatment to stop or reverse the progression of the condition.
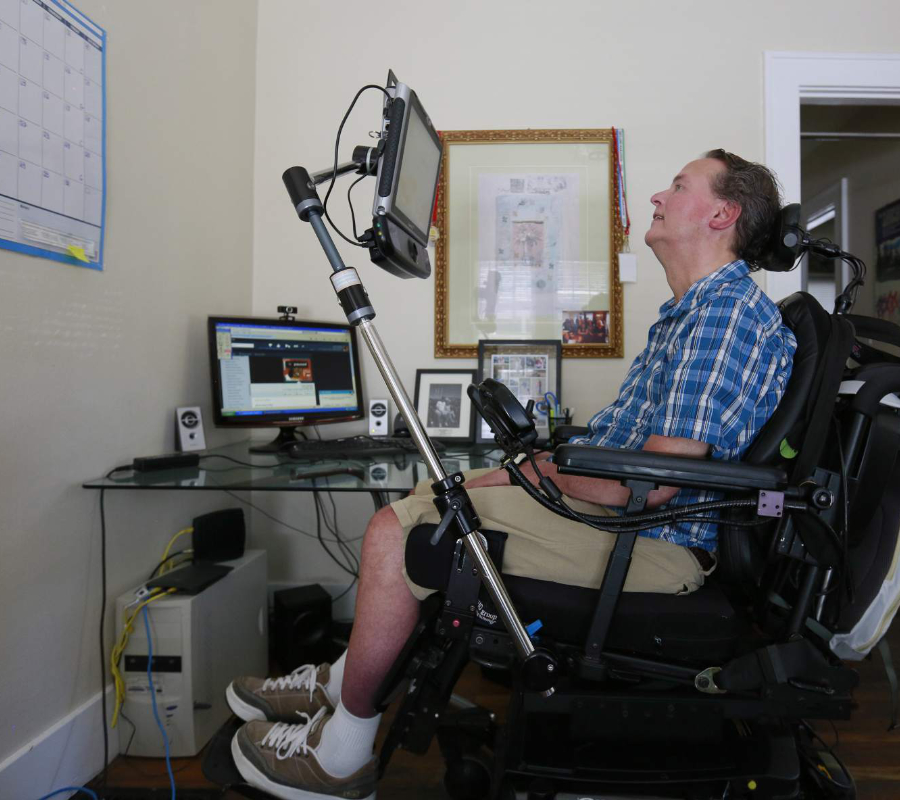
FDA approves the second drug to treat ALS in the U.S.
There are some medications, though, to treat some of the symptoms of ALS. In Japan, edaravone was recently approved to treat ALS, which prompted the DEA to approve the drug in the U.S.
“After learning about the use of edaravone to treat ALS in Japan, we rapidly engaged with the drug developer about filing a marketing application in the United States,” said Eric Bastings, deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research.
According to Bastings, it’s the first new treatment approved by the FDA to treat ALS in many years, and they are pleased that people with the condition will now have an additional option.
According to the NINDS, early symptoms of ALS include muscle weakness or stiffness. As the disease gradually worsens, individuals lose their strength and the ability to move, speak, eat, and even breath.
The Centers for Disease Control and Prevention estimate that between 14,000 and 15,000 Americans have ALS. There are several potential risk factors for ALS such as age, gender and race, and ethnicity.
ALS can strike at any age, but usually, symptoms develop between the ages of 55 and 75. Men are more likely than women to develop the disease. The CDC noted that ALS is most likely to affect Caucasians and non-Hispanics.
Although is still unknown what causes ALS, researchers suggest the disease can be caused due to genetic or environmental factors. In 1993 the NINDS discovered that mutations in the SOD1 gene were linked with some cases of familial ALS.
Then, in 2011 scientists found that a defect in the C90RF72 is present in a significant number of individuals with ALS, as well as in people with a type of frontotemporal dementia. Scientists also link environmental factors to ALS, such as exposure to toxins during warfare, or strenuous physical activity, as probable causes why some veterans or athletes develop the disease.
Radivaca improves daily functioning in patients with ALS
Radicava is an intravenous infusion provided by a health care professional. It is administered with an initial treatment cycle of daily doses for 14 days, followed by 14 days of no dosing. According to the FDA, the efficacy of edaravone to treat ALS was proved in a six-month clinical trial that took place in Japan.
In the trial, 137 participants were randomized to either receive edaravone or placebo, and researchers noted that by week 24, participants receiving edaravone showed better results when compared to participants who received placebo.
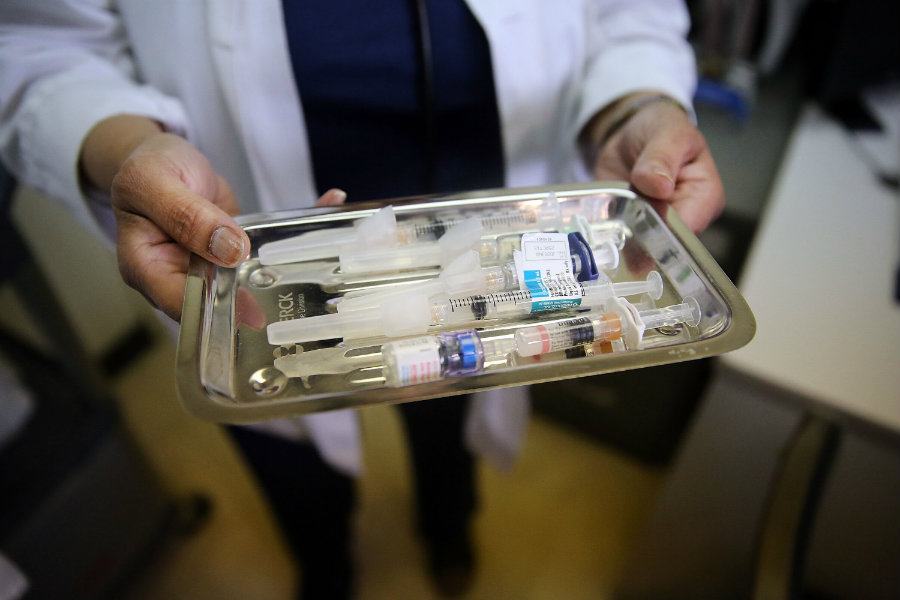
The FDA noted that the most common adverse reactions reported in the clinical trial in patients who received edaravone were bruising (contusion) and gait disturbance. They warned that Radivaca is also associated with serious risks that require immediate medical care, like swelling, hives, shortness of breath, as well as allergic reactions to sodium bisulfite, which is an ingredient in the drug. The FDA granted the drug orphan drug designation, which is intended to provide incentives to assist and encourage the development of drugs for rare or unusual diseases.
The Ice Bucket Challenge helped ALS research
ALS became widely known in 2014 when the Ice Bucket Challenge went viral. The challenge, which consists on dumping a bucket of icy water on top of one’s head, was invented as a way to promote awareness for ALS and to raise money for research regarding the disease.
When a person conducted the Ice Bucket Challenge, such person nominated another to do the challenge and to donate to the ALS Association. Over 17 million people uploaded Ice Bucket Challenge videos to social media, and 2.5 million people donated money to the ALS Association, raising more than $115 million in August 2014.

Source: FDA
